Good quality mining reagent benzohydroxamic acid (BHA) cas 495-18-1 price for sale
Benzohydroxamic acid (BHA) is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents.
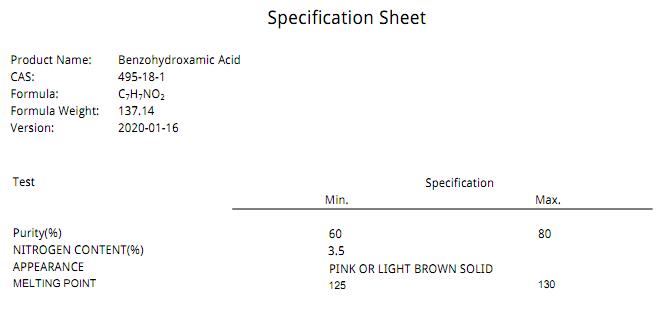
benzohydroxamic acid (BHA) cas 495-18-1
MF: C7H7NO2
MW: 137.14
EINECS: 207-797-6
Melting point 126-130 °C(lit.)
Boiling point 251.96°C (rough estimate)
density 1.2528 (rough estimate)
Form pink or light brown solid
benzohydroxamic acid (BHA) cas 495-18-1
Benzhydroxamic acid (BHA) is used as precursor in the synthesis of novel mono-anionic and di-anionic hydroxamato complexes by reacting with BiPh 3 and Bi(O(t)Bu) 3, which has anti-bacterial activity against helicobacter pylori. It is used in the photometric determination of trace amounts of vanadium in alloy steels by making mixed-ligand vanadium chelates with ammonium thiocyanate.
Sample
Available
Package
1kg per bag, 25kg per drum, or as you required.
Storage
Store the container tightly closed in a dry, cool and well-ventilated place.

Product recommendation
- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Irish
- Greek
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
- Kinyarwanda
- Tatar
- Oriya
- Turkmen
- Uyghur